
- Enseignant: Gökce Dogu
- Enseignant: Miguel Micaelo Bengala
- Enseignant: Michael Pecka
- Enseignant: Laura Busse
- Enseignant: Steffen Katzner
- Enseignant: Lars Kunz
Content: This seminar focuses on current research investigating how neural circuits generate behavior at a mechanistic level. Students will explore how defined neuronal populations and their interactions give rise to specific behavioral outputs. Topics may include circuit-level analyses of navigation, social interaction, sensorimotor transformations, and internal state-dependent behavior. Through critical reading of recent primary literature, students will independently research a selected topic and present their findings in an oral presentation. The course emphasizes clear scientific communication, critical analysis, and active discussion of experimental approaches and interpretations.
Learning Outcome: Students will develop a mechanistic understanding of how neural circuits guide behavior based on current experimental findings in systems neuroscience. They will enhance their skills in reading and critically evaluating primary research articles, with a focus on circuit-level analysis and experimental design. Students will also strengthen their ability to communicate complex scientific ideas through oral presentation and group discussion.
- Enseignant: Alexander Kaiser
- Enseignant: Anna Schröder
- Enseignant: Cornelia Kopp-Scheinpflug
- Enseignant: Lars Kunz
- Enseignant: Michael Pecka
- Enseignant: Alexander Kaiser
- Enseignant: Anton Sirota
Welcome to the forum Teaching Coordination!
- Enseignant: Alexander Kaiser
- Enseignant: José Alcami Ayerbe
- Enseignant: Manfred Gahr
- Enseignant: Stefan Leitner
Goals and learning outcomes
(All goals and learning outcomes will have a strong focus on brain and CNS)
- Introduction to stem cell biology and regenerative medicine
- Understand and acquire knowledge in the field of cells and stem cell biology (e.g., history of the field, stem cell types and sources, characterization, identification, isolation, cellular sorting, genetic modification)
- Ethical aspects of stem cell research and stem cell therapy
- Understand and acquire knowledge about the ethical issues related to stem cell-based therapies (e.g., embryonic stem cells vs iPSCs), and adult stem cells as a possible solution (iPSC reprogramming and differentiation, in vivo direct reprogramming)
- Application of stem cell technologies
- Understand and acquire knowledge about the potential application of cells and stem cell technologies (e.g, drug screening, lab on a chip, disease modeling)
- Technical and practical difficulties of stem cell therapies
- Understand and acquire knowledge about the technical issues related with cell-based therapies (e.g., immune activation, transplant rejection, theratoma formation) and possible novel solutions (e.g., development and use of novel biomaterials and bioscaffols)
- Stem cell bioactive derivates as a potential alternative of stem cell administration in regenerative medicine
- Understand and acquire knowledge about stem cell- derived bioactive derivates as an alternative to traditional cell-based therapies (e.g., extracellular vesicles and exosomes)
- Regeneative medicine I
- Understand and acquire knowledge about the latest achievments and results in vitro, in vivo, and human clinical trials using stem cells derived and stem cells based tools
- Regenerative medicine II
- Understand and acquire knowledge about the use of stem cells therapy for treatment of the most common neurodegenerative and CNS diseases (e.g., AD, PD, HD, Spinal cord regeneration, Stroke)
- Special attention to the 60-years history of cell therapy for the treatment of Parkinson’s disease
Teaching method
Lectures, students presentation and discussions
- Enseignant: Lena Burbulla
- Enseignant: Francesco Gubinelli
- Enseignant: Alexander Kaiser
- Enseignant: Alexander Kaiser
- Enseignant: Wiktor Mlynarski
- Enseignant: Anton Sirota
- Enseignant: Alexander Kaiser
- Enseignant: Arash Shahidi
- Enseignant: Anton Sirota
Course Content: This course provides hands-on training in modern videography techniques and electrophysiology methods used in behavioral and sensory neuroscience research. Students will gain practical skills in subject tracking, kinematic feature extraction, behavioral segmentation, and behavioral classification, along with essential techniques for preprocessing and analyzing electrophysiological data. The course will also focus on establishing connections between behavioral and neural variables to infer the understanding of underlying mechanisms.
Learning Outcome: Throughout the course, students will actively engage in practical exercises, including data collection, analysis, and interpretation. They will also have opportunities to work with state-of-the-art videography and electrophysiology equipment and software, gaining proficiency in their application. By the end of the course, students will have a comprehensive understanding of modern videography and electrophysiology techniques and the ability to relate behavioral and neural variables effectively.
Prerequisites: Basic knowledge of neuroscience, statistics, and programming is recommended. Familiarity with videography and electrophysiology concepts will be advantageous but not mandatory.
- Enseignant: Justin Graboski
- Enseignant: Alexander Kaiser
- Enseignant: Anton Sirota
Content: We will discuss articles on computational neuroscience ranging from cellular to network computation. Every student will present one article on the topic of their choice on cell and network computation. All students will have to read one article presented by other students and actively participate in a discussion. We will discuss in depth each article and proceed to an interactive 'review' where we will simulate an article revision, and take the roles of editors and reviewers. Constructive feedback on the presentations will further be given to help improving presentation skills.
Evaluation will consist of the article presentation (50%) and the contribution to the discussion of other articles during the course (50%). A basic knowledge of neuroscience is required. The course mainly targets Master and PhD students.
- Enseignant: José Alcami Ayerbe
- Enseignant: Alexander Kaiser
Content: We will discuss a comprehensive and
original approach to computational tasks performed by the brain based on
the book "Principles of Neural Design" by Simon Laughlin and Peter
Sterling. Students will read the book, and one chapter per week will be
presented by a student and critically discussed. Evaluation will consist
of the chapter presented (50%) and the discussion along the course of
other chapters (50%).
- Enseignant: José Alcami Ayerbe
- Enseignant: Caroline Geisler
- Enseignant: Alexander Kaiser
Content: This seminar introduces recent data
analysis methods highlighted in current research papers. Using Python,
participants will actively implement these techniques, gaining first
hand experience in application of the methods to the real data and
interpreting results. The foundational theories behind these methods
will be discussed, referencing established analytical texts. We'll
particularly focus on analysis methods regarding time series data,
dimensionality reduction, and dynamical systems.
Learning Outcomes: Grasp and apply contemporary data analysis methods from recent research.
Efficiently utilize Python to implement and validate these techniques.
Comprehend the basic theoretical principles underpinning these methods.
Acquire the capability to assess and modify these techniques for specific research objectives.

- Enseignant: Alexander Kaiser
- Enseignant: Arash Shahidi
- Enseignant: Anton Sirota
- Enseignant: Daniela Billenstein
- Enseignant: Alexander Kaiser
- Enseignant: David Keays
Hallo zusammen!
Dieser Moodlekurs dient nur dazu, unsere Informationen an alle Teilnehmer an einer Stelle zu bündeln. So gehen keine Infos in Emailwüsten verloren, und auch potentielle Nachrücker können schnell und einfach alles an einer Stelle finden.

- Enseignant: Sven Schörnich
- Enseignant: Andreas Zahn
- Enseignant: Alexander Kaiser
- Enseignant: Anton Sirota
Seminar
Wed 11:30 - 13:00h
B01.015
Description:
Schedule:
- Enseignant: Alexander Kaiser
- Enseignant: Anton Sirota
Content: Organoids represent an emerging model system technology that allows the growth and analysis of human organ-like tissues in the dish. Brain organoids in particular have proven valuable for research due to the difficulty of investigating human brain development experimentally. In this 2-week block course, the participants will learn how to work in cell culture as well as grow and analyze brain organoids themselves. The course consists of a theoretical and a practical part.
Learning outcome: During the theoretical morning lectures, the students will learn the theoretical basics of stem cell and organoid culture as well as present a recent paper on organoid technologies themselves. During the practical parts in the afternoon, the students will acquire hands-on experience in organoid culture. They will be introduced to good working practices that are required in a sterile stem cell lab. Consequently, they will learn how to culture and passage stem cells as well as grow their own organoids using the original brain organoid protocol (Lancaster and Knoblich, 2014). Finally, the students will learn how to analyze organoids histologically.
3 ECTS; LMU Biocenter Neurobiology- Enseignant: Patrick Heisterkamp
- Enseignant: Alexander Kaiser
- Enseignant: Thamari Kapuruge
- Enseignant: David Keays
Content:
Students in this course will be introduced to fundamental experimental methods in neuroscience, e.g. neuroanatomy, behavior and neurophysiology. In several experimental sessions they will learn the basic working principles of imaging techniques, behavioral and electrophysiological setups, as well as record, analyze and interpret respective datasets.
Learning outcomes:
After successful completion of this module, students will have covered the most fundamental neuroscientific data acquisition techniques, as well as the basics of thoughtful and critical interrogation and interpretation of the recorded data.
- Enseignant: Laura Busse
- Enseignant: Alexander Kaiser
- Enseignant: David Keays
- Enseignant: Cornelia Kopp-Scheinpflug
- Enseignant: Lars Kunz
- Enseignant: Simon Nimpf
- Enseignant: Michael Pecka
- Enseignant: Evgeny Resnik
- Enseignant: Anna Schröder
- Enseignant: Anton Sirota
- Enseignant: Anton Sumser
In this lab you will obtain intracellular recordings from the neurons of the medicinal leech (Hirudo medicinalis).
By the end of this lab you will be able to do the following:
- Understand the importance of AD/DA conversions, sampling rates and amplification gains.
- Dissect a leech and identify segmental ganglia.
- Use a micromanipulator to position glass recording electrodes (microelectrodes) over the leech ganglion.
- Make intracellular recordings from leech neurons.
- Deliver current injections through the electrode using an intracellular amplifier and the PowerLab.
- Identify different at least 5 types of neurons (Retzius cells, T cells, N cells, P cells and motor neurons).
- Stimulate the sensory neurons (T cells) that are responsive to touch on the skin.
- Attempt paired recordings of two Retzius cells and observe the gap-junction coupling.
Additionally you will learn how to to run neuronal simulations.
- Enseignant: Alexander Kaiser
- Enseignant: Cornelia Kopp-Scheinpflug
- Enseignant: Kay Thurley
- Enseignant: Daniela Billenstein
- Enseignant: Alexander Kaiser
- Enseignant: David Keays
- Enseignant: Simon Nimpf
TRR 274 is a research consortium with scientists from Munich and Göttingen, who collaborate to investigate the checkpoints that determine CNS recovery. Learn more on our website.
Course content: The CNS is a terminally differentiated tissue, where any insult carries a heightened risk - yet the tissue response to these insults is variable and can range from irreversible destruction to almost complete recovery. The rules that instruct these divergent outcomes are still unknown. The topic of this lecture series is the biology of the multicellular response that determines recovery after CNS injury. We will focus on the multi-scale, spatio-temporal cell biology in different models of CNS injury. The participants will not only be introduced to principles of CNS recovery, but also the new imaging technology that is necessary to study the biology. Another focus will be put on neuronal, glial and immunological aspects of CNS recovery/damage.
Learning objectives:
- Introduction into various inflammatory, traumatic, metabolic or ischemic models and their various mechanisms that determine the balance between reconstitution and scarring (e.g. multiple sclerosis and inflammation induced CNS damage, axonal injury and loss, spinal cord injury, stroke)
- Introduction into imaging approaches in neuroscience (specifically electron microscopy techniques to understand ultrastructural details of CNS tissue and in vivo imaging approaches)
- Principles of CNS recovery from a neuronal, glial and immunological perspective (e.g. Remyelination, axonal regeneration, role of inflammatory cells in injury and recovery)
- Presentation of current research findings in the recovery of the CNS recovery (therapeutic approaches and molecular targets)
2 ECTS; via Zoom; registration per email required at adinda.wens@med.uni-goettingen.de until 10.10.2025.

- Enseignant: Camilla Giudici
- Enseignant: Alexander Kaiser
- Enseignant: Adinda Wens
Willkommen zur Übung "Methoden der Physiologie - Teile Human- und Tier-Physiologie"!
Die
Übung besteht aus sechs Kursteilen, die über den November und Dezember hinweg
durchgeführt werden.
- Enseignant: José Alcami Ayerbe
- Enseignant: Oliver Behrend
- Enseignant: Sebastian Bias
- Enseignant: Wolfgang Enard
- Enseignant: Franziska Fröhlich
- Enseignant: Johanna Geuder
- Enseignant: Antonia Keßler
- Enseignant: Cornelia Kopp-Scheinpflug
- Enseignant: Lars Kunz
- Enseignant: Josef Mautner
- Enseignant: Michael Pecka
- Enseignant: Felix Pförtner
- Enseignant: Daniel Richter
- Enseignant: Anton Sumser
- Enseignant: Beate Vieth
- Enseignant: Ming Zhao
- Enseignant: José Alcami Ayerbe
- Enseignant: Richard Merrill
- Making electrodes
- Handling and training animals
- Implanting electrodes into the brain
- Recording extracellular signals
- Origin of extracellular potentials
- Physical principles of extracellular recording
- Practical aspects of extracellular recordings
- Preparing ephys data for analysis
- Analysis of LFP signals, detection and quantification of low and high frequency oscillations
- Sorting of extracellular spikes
- Analysis of spike trains
- Current-source density (CSD) analysis
- Potential caveats and pitfalls in analysis of LFP signals
For each topic I provide first a presentation with theoretical introduction. Then we switch to Matlab where I explain how to implement a particular analysis using a well-commented demo script. At the end the participants can try the explained analysis on their own using the provided list of exercises as a guideline.
Programming skills or prior Matlab knowledge is helpful, but not necessary. No need for a powerful PC or laptop, or Matlab license, because all the work is done remotely on the lab server. Fast enough internet connection is desirable.
Content: The course is intended for Master and PhD students interested in systems neuroscience and willing to learn state-of-the-art extracellular recording techniques and analysis of the electrophysiological data. The course includes both theoretical introduction into all the aspects of the extracellular recording and hands-on practicum on recordings with tetrodes or silicon probes of local field potentials and multiple single neurons in freely moving rats or mice. Training will include animal handling and training, preparing electrodes, brain surgery, recording in typical behavioral tasks, data processing, spike sorting and typical data analysis of local field potentials and spike trains in Matlab.
Acquired skills: After participating in the course, the students will learn about modern extracellular recording techniques, the origin and interpretation of extracellular signals, application of the techniques to spatial navigation and learning research. They will be familiarized with the terminology relevant to the electrophysiological experiments. Under close supervision, the students will go through all the principle steps of a typical experiment themselves. At the end they will be able to handle and train animals, make tetrodes and implant them into the brain, operate an acquisition system to record extracellular signals, visually explore and evaluate quality of the recorded data, prepare the data for further analysis, do spike sorting, program Matlab scripts to perform typical spectral and spike train analysis. The acquired knowledges and skills can be directly applied to specific scientific projects the students are already working on or plan to work on in the future.
| 3 ECTS; 2 week full-day (9:00-18:00 s.t.) block course; |
- Enseignant: Evgeny Resnik
Inhalt
In der Vorlesung werden theoretische und praktische Grundkenntnisse in Tier- und Humanphysiologie vermittelt. Die Vorlesung führt ein in grundlegende Aspekte der Tierphysiologie, dies sind insbesondere: Osmoregulation, Muskelphysiologie, Herz- und Kreislaufphysiologie, Ionentransport über Membranen und Nernst-Gleichung, Atemphysiologie, Sehen, Hören und EEG.
Qualifikationsziele
Lerninhalte sind theoretische Grundlagen der Physiologie der Tiere und des Menschen. Die Studierenden beherrschen die Inhalte der Vorlesungen und sind zum Wissenstransfer auf aktuelle Probleme fähig.
- Enseignant: Otto Albrecht
- Enseignant: José Alcami Ayerbe
- Enseignant: Oliver Behrend
- Enseignant: Wolfgang Enard
- Enseignant: Cornelia Kopp-Scheinpflug
- Enseignant: Lars Kunz
- Enseignant: Josef Mautner
- Enseignant: Michael Pecka
- Enseignant: Sven Schörnich
- Enseignant: Anton Sumser
Content: The course provides a comprehensive view on the most modern molecular techniques employed to tackle fundamental questions in neurobiology. A particular emphasis will be laid on the use of modern tools to manipulate DNA, reprogram cellular identity and trace brain connectivity, in health and disease. Learning outcome: Understand how modern techniques can help unravelling unsolved questions in molecular neurobiology. |

- Enseignant: Alexander Kaiser
- Enseignant: Giacomo Masserdotti
- Enseignant: Otto Albrecht
- Enseignant: Hans Straka
- Enseignant: Oliver Behrend
- Enseignant: Benedikt Grothe
- Enseignant: Lars Kunz
The course consists of a lecture and excercise to deepen knowledge in several neurohistological methods in mammals. Topics include stereotaxis, fixation and preparation of nervous tissue, sectioning, immunostaining, tracing methods, analysing of stained sections with bright field, epifluorescence and confocal laser scanning microscopy. Furthermore, methods for preparing high quality images from multi-immunostainings are applied to prepared sections. Finally, the students will analyse and discuss their results in respect to related publications and prepare a presentation. The students work in groups of 2-3 on individual projects and present the outcome within the class.

- Enseignant: Alexander Kaiser
- Enseignant: Oskar Markkula
- Enseignant: Anna Schröder
- Enseignant: Luna Amanda Studer
- Enseignant: José Alcami Ayerbe
- Enseignant: Alexander Kaiser
- Enseignant: Steffen Katzner
- Enseignant: Ann Kotkat
The lecture Systems Neuroscience 1 addresses the principles of
sensory processing, the transduction of adequate stimuli, and ensuing sensory-motor interactions. A detailed description of the peripheral and central
stages of each specific sensory system is accompanied by theoretical
concepts of the underlying neuronal processing. The lecture is given
weekly and requires regular attendance and a final exam. The
following sensory systems are covered by participating lecturers:
-The mechanosensory lateral line system of aquatic animals and its role in the detection, identification and localisation of objects on the water surface or within the water body
-Electroreception: peripheral and central properties of independently evolved systems, and the systems’ role in object detection, orientation, and communication
-The ontogenesis, organization and plasticity of the vestibular system and general aspects of sensory-motor interaction
-Properties of diverse magnetoreceptive systems-Principles of several chemoreceptive systems, peripheral and central processes of the gustatory and the olfactory system, multimodal interactions
-Peripheral and central stages of somatosensory systems, and their function
-Mechanisms of pain, and temperature perception
- Enseignant: Oliver Behrend
- Enseignant: Daniela Billenstein
- Enseignant: Alexander Kaiser
- Enseignant: David Keays
- Enseignant: Simon Nimpf
- Enseignant: Anton Sumser
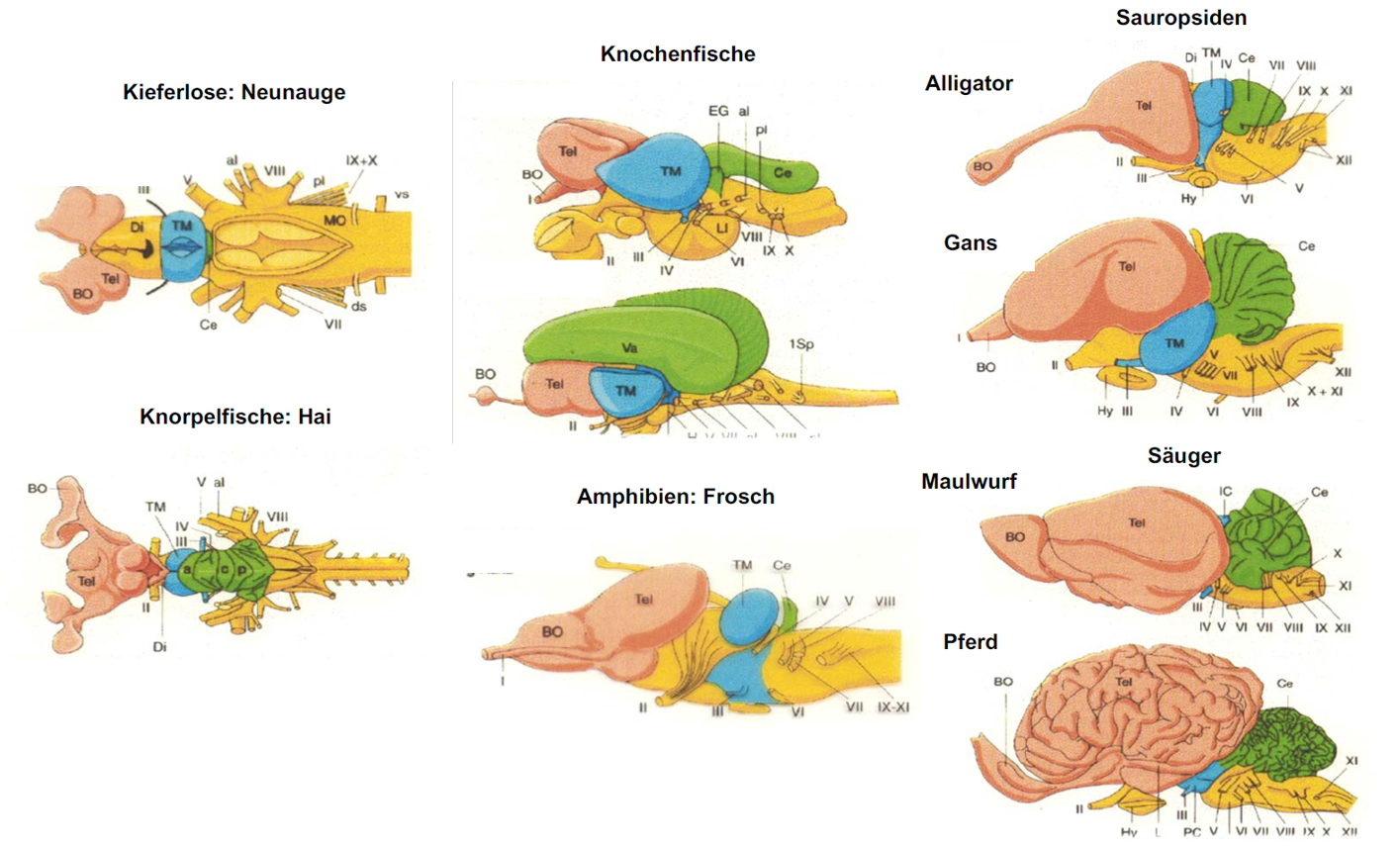
- Enseignant: Benedikt Grothe
- Enseignant: Alexander Kaiser
- Enseignant: Lars Kunz
- Enseignant: Maria Sanchez Gonzalez
- Enseignant: Anton Sumser
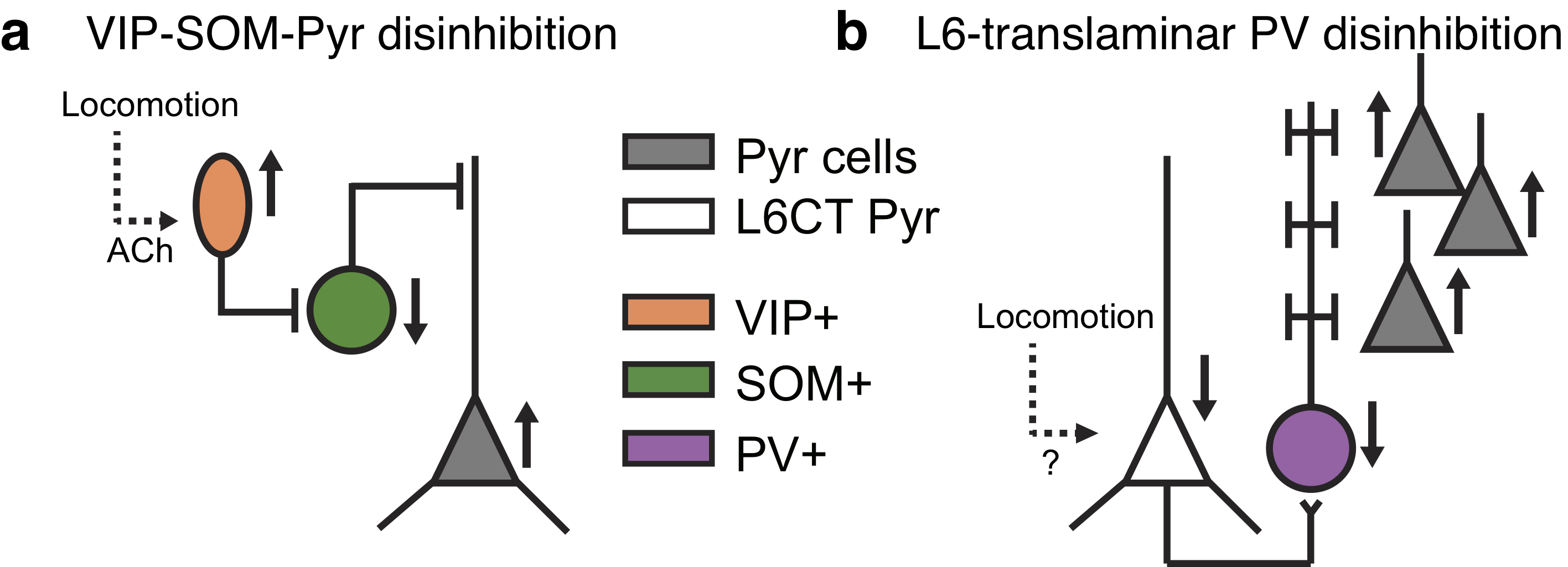
- Enseignant: Laura Busse
- Enseignant: Steffen Katzner
The lecture provides an introduction to fundamental principles in Neuroscience. The lecture consists of 26 topics which are organized in 4 blocks:
(1) Cellular Molecular, Synapses, Electrophysiology, Networks
(2) Nervous System Development
(3) Comparative Neurobiology and Evolution of the Brain
(4) Plasticity: Learning and Memory
We are looking forward to having you in Fundamentals in Neuroscience I!
Responsible: Laura Busse (busse at bio.lmu.de)
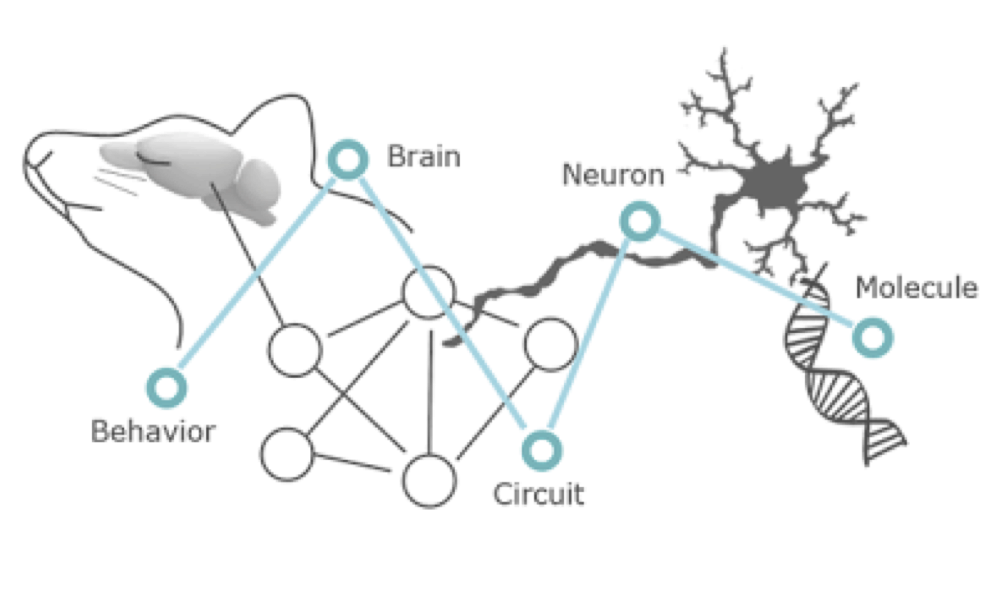
- Enseignant: José Alcami Ayerbe
- Enseignant: Laura Busse
- Enseignant: Benedikt Grothe
- Enseignant: Alexander Kaiser
- Enseignant: Steffen Katzner
- Enseignant: Maria Sanchez Gonzalez
- Enseignant: Anna Schröder
- Enseignant: Anton Sirota
- Enseignant: Anton Sumser
The WP10 Biomedical Neuroscience Module offers a comprehensive introduction to clinical neuroscience research. The module consists of two parts:
- WP10.1: Lecture series every Thursday, 17.00-18.30h, online via Zoom-Meetings, exam, 2 ECTS.
- WP10.2: Seminar to the lecture
series every Friday, 09.00-10.30h, online via
Zoom-Meetings, presentation, 1 ECTS.
- Enseignant: Filipp Filippopulos
- Enseignant: Peter Graf zu Eulenburg
- Enseignant: Alexander Kaiser
Content
In this seminar current research in neurobiology under the umbrella topic "Sensing in Action" are discussed. We will approach the topic from many perspectives - e.g. comparative, behavioral, systems, cellular and molecular and will discuss findings in broad context. |
Learning outcomes
The seminar will introduce students to the current state-of-the-art in neuobiology, reading and condensing scientific literature, as well as presenting and discussing neurobiological findings. |
Structure
Each student will be assigned to a publication, which should be read in depth and summarized in a presentation for the group. Afterwards, the paper, findings and presentation style will be discussed by the group with support of the supervisors. |
3 ECTS points

- Enseignant: Laura Busse
- Enseignant: Alexander Kaiser
- Enseignant: Lars Kunz
- Enseignant: Michael Pecka
- Enseignant: Sven Schörnich
- Enseignant: Anna Schröder
- Enseignant: Anton Sirota
- Enseignant: Anton Sumser
Content
This series of lectures offers detailed insight into the fundamentals underlying mammalian - including human - vision and hearing. The lectures cover a wide range of topics including basics, main neuronal structures and processing principles within the visual and auditory pathway as well as interesting psychophysical phenomena.
Learning outcomes
Knowledge of principles of auditory and visual neuronal processing, including behavioural consequences. The students should be able to outline these basic principles and transfer their knowledge into an exam situation. Students will obtain the fundamental knowledge required to participate in further specialized courses of the Master Program and will acquire the basic knowledge prerequisite to physiological research.
- Enseignant: Oliver Behrend
- Enseignant: Laura Busse
- Enseignant: Benedikt Grothe
- Enseignant: Alexander Kaiser
- Enseignant: Steffen Katzner
- Enseignant: Michael Pecka
- Enseignant: Ruben Portugues Peters
- Enseignant: Sven Schörnich